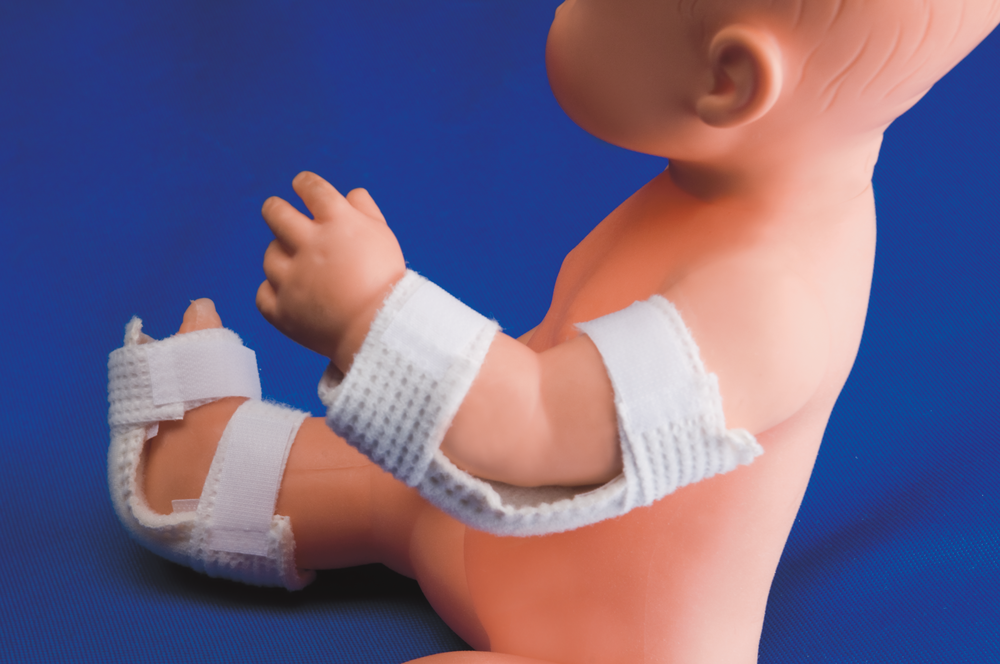
NEONATAL WRIST - ANKLE HOLDER FOR INTRAVENOUS THERAPY
Indicated to immobilize and protect the patients punction areas, used in life support areas, emergency services, intensive care unit, etc.
It holds the I.V. and intra-arterial therapy elements to the patient’s extremities to avoid any brisk movement.
Composition:
Support base : Aluminium 100%.
Support cover: Inside padding in polyurethane foam with a velours layer.
Strip: Velours and non-woven polyester 100%
All materials are Medical grade.
Instructions for use
CTBB202N: Newborn
CTBB202P: Pediatric
CTBB202T: Teeneger
CTBB202A: Adult
It holds the I.V. and intra-arterial therapy elements to the patient’s extremities to avoid any brisk movement.
Composition:
Support base : Aluminium 100%.
Support cover: Inside padding in polyurethane foam with a velours layer.
Strip: Velours and non-woven polyester 100%
All materials are Medical grade.
Instructions for use
- Place the support under the extremity bending it until it fits to the area.
- Fix it passing the strips around the extremity and hold it through Velcro closure.
CTBB202N: Newborn
CTBB202P: Pediatric
CTBB202T: Teeneger
CTBB202A: Adult
| CARACTERISTICS | ADVANTAGES | PROFITS |
| Flexible and light | Guarantees a perfect adaptation. | The flexibility offers a good adaptation. |
| Breathable material | No skin irritable, antiallergenic. It allows the breathing of the skin, humidity, maceration and avoids the compression. |
Soft and secure support. |
| Velcro® closure | Adjustable and safe. | Flexible closure. Safety fastening. |
| Washable | Reusable with the same patient, not for others patients. If the product is not contaminated by infectious agents can be cleaned with mild soap and water.. | Avoid the risk of cross contamination. |
| Useful | Easy and fast. Velcro® closure for fast action to put on and remove the support. |
Saves time for nursing staff. |
| CODE | MODEL | LENGHT (cm) | WIDTH. (cm) | UNIT (per box) | DIMENSION (per box) | WEIGHT (per box) |
| CTBB202N | Neonatal | 10 | 4 | 30 | 20x15x15 | 0,44 |
| CTBB202P | Pediatric | 16 | 6 | 30 | 20x15x15 | 0,913 |
| CTBB202T | Adolescent | 18 | 6 | 30 | 31x21x23 | 1,6 |
| CTBB202A | Adut | 26 | 8 | 30 | 31x21x23 | 2,25 |

 English
English Italiano
Italiano